
Antioxidant BHT 264
CAS:128-37-0
Purity:99%
Contact Now
We will contact you as soon as possible
Your Location:Home >Products >Chemical Reagents >2979-19-3
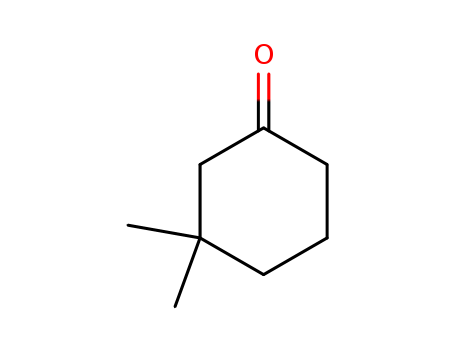

Product Details
|
Uses |
An interest in the boll weevil sex attractants prompted an investigation into alternative syntheses of the starting material, 3, 3-dimethylcyclohexanone. |
|
Synthesis Reference(s) |
The Journal of Organic Chemistry, 40, p. 3619, 1975 DOI: 10.1021/jo00912a040 |
InChI:InChI=1/C8H14O/c1-8(2)5-3-4-7(9)6-8/h3-6H2,1-2H3
-
-
Irradiation of unsaturated bicyclo[4.1.0...
-
-
Allele-specific enzyme inhibitors are po...
-
The synthesis of lysine analogues wherei...
The treatment of α,β-unsaturated carbony...
The treatment of α,β-unsaturated carbony...
The present application relates to the f...
An efficient oxidation and functionaliza...
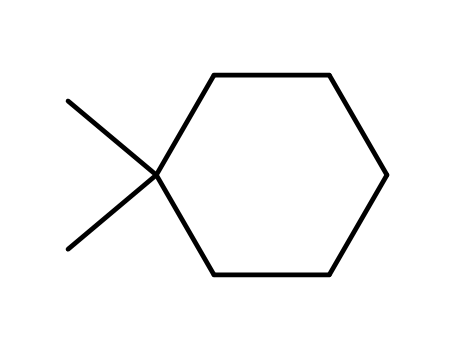
1,1-dimethylcyclohexane

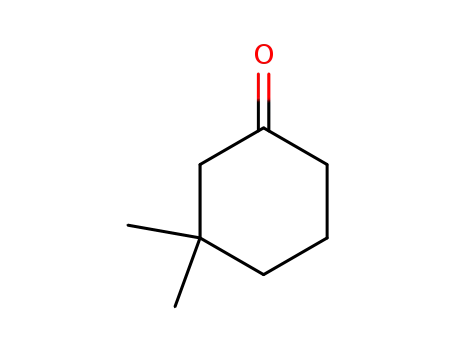
3,3-dimethylcyclohexanone

3,3-dimethylcyclohexanol

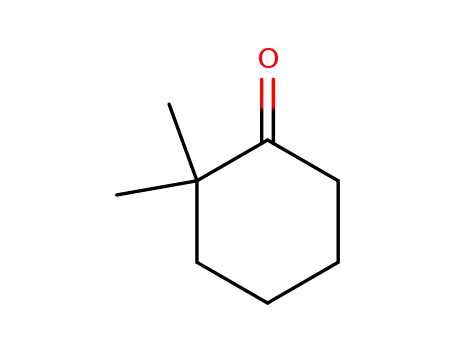
2,2-dimethyl-cyclohexanone

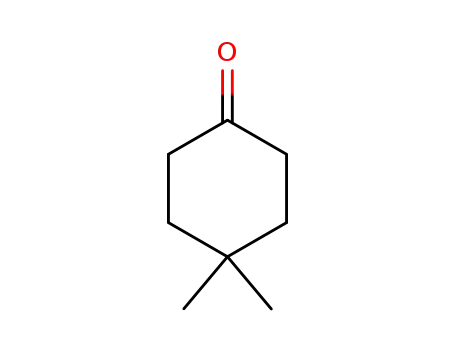
4,4-dimethylcyclohexane-1-one
| Conditions | Yield |
|---|---|
|
With
[(S,S)-Fe(1,1'-bis((5-(2,6-bis(trifluoromethyl)phenyl)pyridin-2-yl)methyl)-2,2'-bipyrrolidine)(acetonitrile)2](SbF6)2 ; dihydrogen peroxide; acetic acid;
In
water; acetonitrile;
at 20 ℃;
for 0.5h;
|
14 %Chromat. 29 %Chromat. 18 %Chromat. 11 %Chromat. |
|
With
[(S,S)-Fe(1,1'-bis((5-(2,6-bis(trifluoromethyl)phenyl)pyridin-2-yl)methyl)-2,2'-bipyrrolidine)(acetonitrile)2](SbF6)2 ; dihydrogen peroxide; acetic acid;
In
water; acetonitrile;
at 20 ℃;
for 0.5h;
|
16 %Chromat. 35 %Chromat. 17 %Chromat. 13 %Chromat. |

1,1-dimethylcyclohexane


3,3-dimethylcyclohexanone


2,2-dimethyl-cyclohexanone


4,4-dimethylcyclohexane-1-one
| Conditions | Yield |
|---|---|
|
With
[FeII(N,N'-(bis(2-pyridylmethyl)-(S,S)-2,2'-bipyrrolidine))(CH3CN)2](SbF6)2; dihydrogen peroxide; acetic acid;
In
acetonitrile;
at 25 ℃;
for 0.5h;
regioselective reaction;
|
21 %Chromat. 32 %Chromat. 17 %Chromat. |
|
With
Λ-[Fe(CF3SO3)2((S,S,R)-BPBPP)]; dihydrogen peroxide; acetic acid;
In
acetonitrile;
Reagent/catalyst;
Overall yield = 68 %Chromat.; regioselective reaction;
|
|
|
With
Fe(triflate)2(1-(6-methyl-2-pyridylmethyl)-4,7-dimethyl-1,4,7-triazacyclononane); dihydrogen peroxide; acetic acid;
In
water; acetonitrile;
at 0 ℃;
for 0.666667h;
Reagent/catalyst;
|
19 %Chromat. 26 %Chromat. 13 %Chromat. |
|
With
tert.-butylhydroperoxide; C19H15F3IO5PS; potassium carbonate;
In
tetrachloromethane; acetonitrile;
at -20 ℃;
for 4h;
Reagent/catalyst;
Solvent;
Overall yield = 79 %Chromat.; regioselective reaction;
Inert atmosphere;
Molecular sieve;
|
|
|
With
[((1S,2,S)-N,N'-dimethyl-N,N'-bis(2-pyridylmethyl)-1,2-cyclohexanediamine)bis(triflate)iron]; dihydrogen peroxide; acetic acid;
In
water; acetonitrile;
at 0 ℃;
for 0.9h;
Reagent/catalyst;
Overall yield = 71 %Chromat.; regioselective reaction;
|
|
|
With
[Fe(CF3SO3)2(N,N’-bis(2-pyridylmethyl)-2,2’-bipyrrolidine)]; dihydrogen peroxide; acetic acid;
In
water; acetonitrile;
at 0 ℃;
for 0.9h;
Reagent/catalyst;
Overall yield = 65 %Chromat.; regioselective reaction;
|
|
|
With
C40H68F6FeN4O6S2Si2; dihydrogen peroxide; acetic acid;
In
water; acetonitrile;
at 0 ℃;
for 0.166667h;
Reagent/catalyst;
Overall yield = 70 %Chromat.;
|
|
|
With
C40H66F6FeN4O6S2Si2; dihydrogen peroxide; acetic acid;
In
water; acetonitrile;
at 0 ℃;
for 0.166667h;
Reagent/catalyst;
Overall yield = 69 %Chromat.;
|
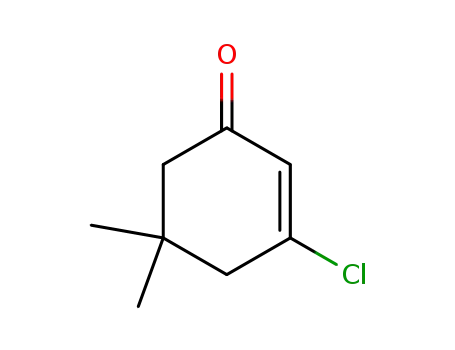
3-chloro-5,5-dimethylcyclohex-2-en-1-one
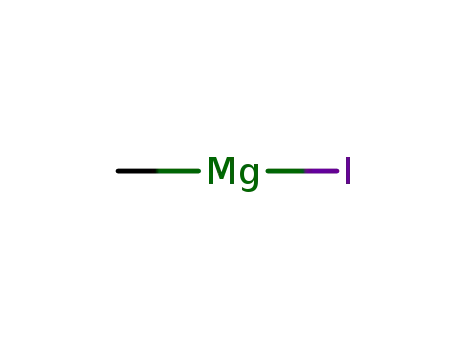
methyl magnesium iodide
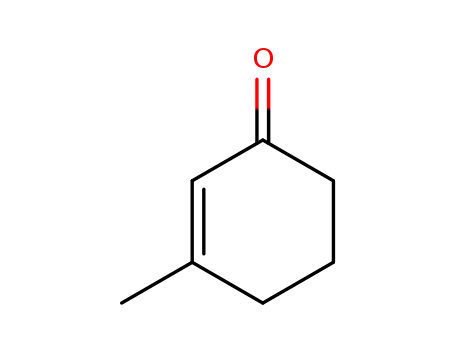
3-methylcyclohexen-2-one
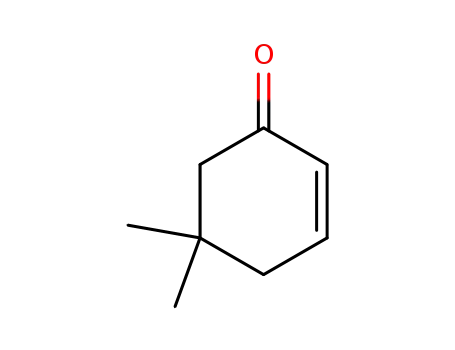
5,5-dimethlycyclohex-2-en-1-one
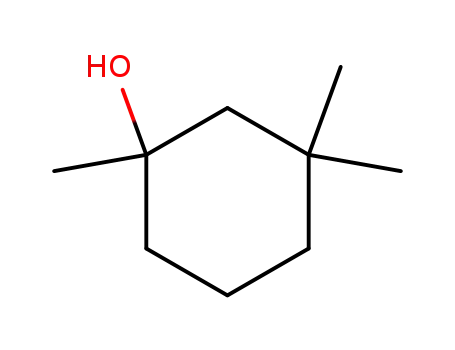
1,3,3-trimethyl-cyclohexan-1-ol
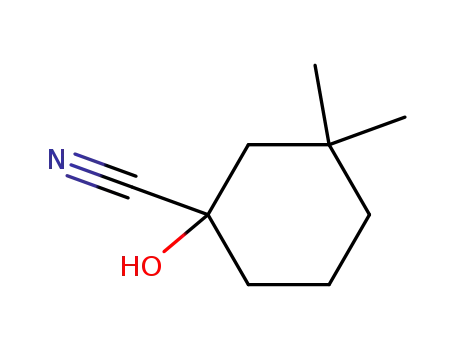
1-hydroxy-3,3-dimethyl-cyclohexanecarbonitrile
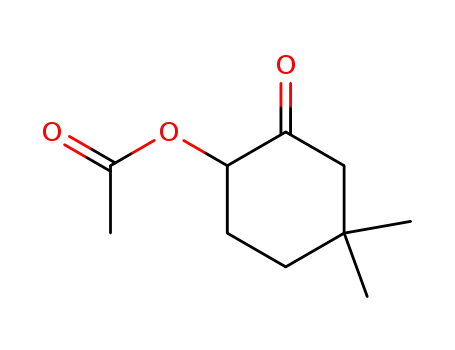
2-acetoxy-5,5-dimethyl-cyclohexanone
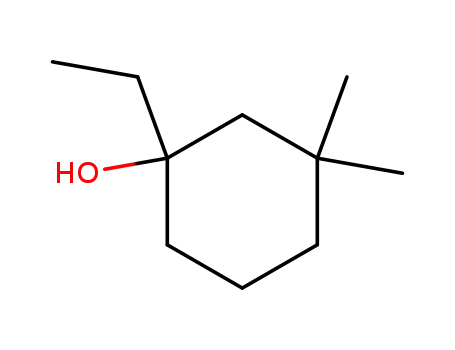
1-ethyl-3,3-dimethyl-cyclohexanol