
Antioxidant BHT 264
CAS:128-37-0
Purity:99%
Contact Now
We will contact you as soon as possible
Your Location:Home >Products >Biochemical Engineering >6610-42-0
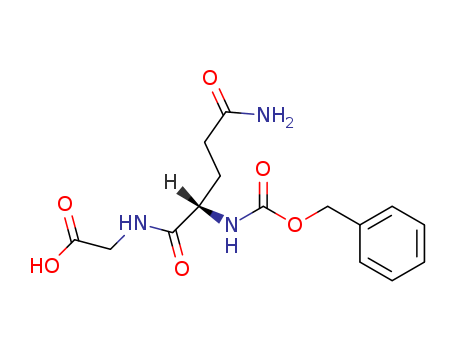

Product Details
|
Uses |
γ-Glutamyl donor substrate used in spectrophotometric determination of transglutaminase (TGase) activity. Z-Gln-Gly was used to enzymatically synthesize N-linked neoglycoproteins. |
|
Biochem/physiol Actions |
N-Benzyloxycarbonyl-L-Glutaminylglycine (Z-Gln-Gly, Z-QG) is used as a substrate to differentiate and characterize transglutaminase(s) (TGase) that catalyzes the post-translational covalent cross-linking of Gln- and Lys-containing peptides. Z-QG supports glutamyl-level cross-linking applications thruough surface modification. |
InChI:InChI=1/C15H19N3O6/c16-12(19)7-6-11(14(22)17-8-13(20)21)18-15(23)24-9-10-4-2-1-3-5-10/h1-5,11H,6-9H2,(H2,16,19)(H,17,22)(H,18,23)(H,20,21)
Several series of dipeptides and tripept...
Cbz-L-Gln

α-N-carbobenzyloxy-L-glutamine-glycine
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 2 steps
1: Et3N; DMAP / acetonitrile / 0.75 h / 20 °C
2: Et3N / acetonitrile; H2O / 1.67 h / 20 °C
With
dmap; triethylamine;
In
water; acetonitrile;
|
|
|
Multi-step reaction with 2 steps
1: PCl3; pyridine
2: acetone; aqueous NaOH
With
pyridine; sodium hydroxide; acetone; phosphorus trichloride;
|
|
|
Multi-step reaction with 2 steps
1: DCC, Et3N
2: aq. NaOH, Py
With
pyridine; sodium hydroxide; triethylamine; dicyclohexyl-carbodiimide;
|
|
|
Multi-step reaction with 2 steps
1: (i) ClCO2Et, Et3N, THF, DMF, (ii) /BRN= 741933/
2: aq. NaOH / dioxane
With
sodium hydroxide;
In
1,4-dioxane;
|
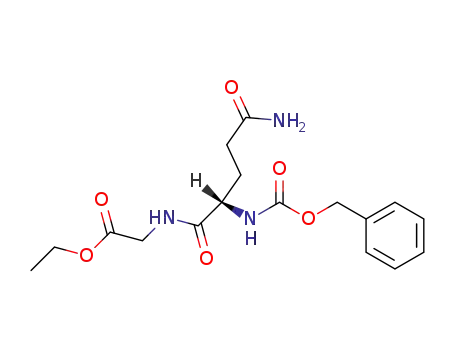
Z-Gln-Gly-OEt


α-N-carbobenzyloxy-L-glutamine-glycine
| Conditions | Yield |
|---|---|
|
With
sodium hydroxide; acetone;
|
|
|
With
sodium hydroxide;
|
|
|
With
sodium hydroxide;
In
1,4-dioxane;
|
|
|
With
pyridine; sodium hydroxide;
|

Z-Gln-Gly-OEt
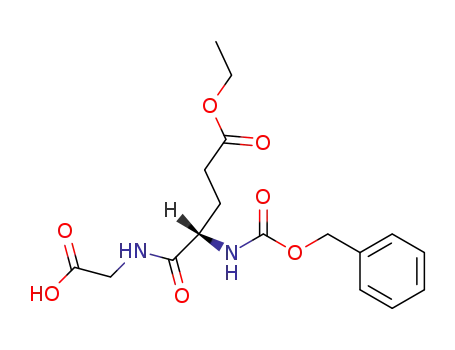
N-(O-ethyl-N-benzyloxycarbonyl-L-α-glutamyl)-glycine
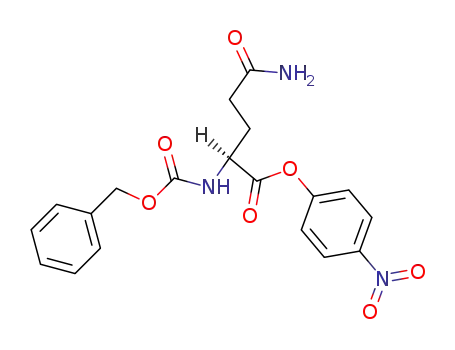
N2-benzyloxycarbonyl-L-glutamine-(4-nitro-phenyl ester)
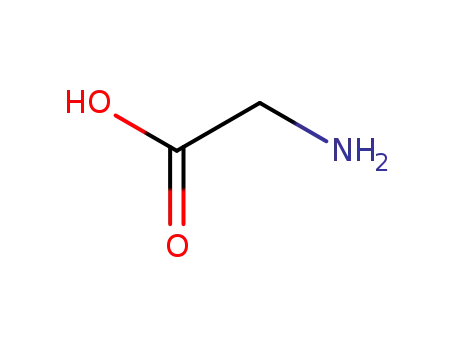
glycine
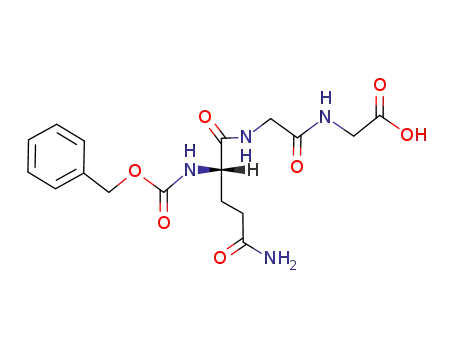
Cbz-L-Gln-Gly-Gly
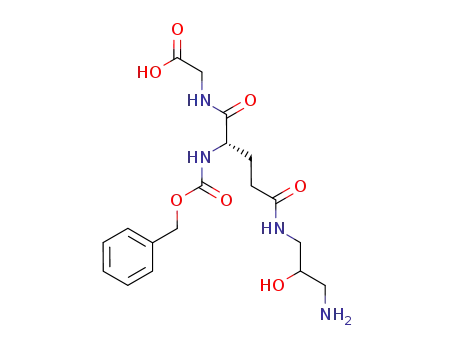
[4-(3-amino-2-hydroxy-propylcarbamoyl)-2-benzyloxycarbonylamino-butyrylamino]-acetic acid
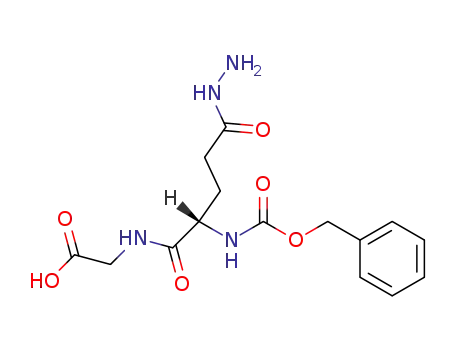
Z-α-L-Glutamyl-(γ-hydrazid)-glycin