
Antioxidant BHT 264
CAS:128-37-0
Purity:99%
Contact Now
We will contact you as soon as possible
Your Location:Home >Products >Analytical Chemistry >6418-56-0
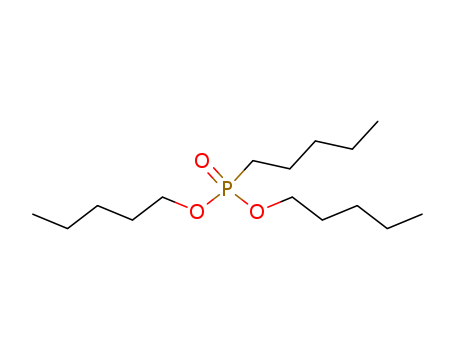

Product Details
|
Purification Methods |
Purify it by three crystallisations of its uranyl nitrate complex from hexane (see tributyl phosphate). It extracts Zr2+ from NaCl solutions. |
InChI:InChI=1/C15H33O3P/c1-4-7-10-13-17-19(16,15-12-9-6-3)18-14-11-8-5-2/h4-15H2,1-3H3
The present paper deals with the retention of U(IV) and U(VI) species, both individually and in 1 :1 mixtures, on some ion-exchange resins of the normal DGA (N,N’,N’-tetra-n-octyldiglycolamide), TEVA- [trialkyl methyl-ammonium nitrate (or chloride)] and UTEVA-type (dipentyl pentylphosphonate), in different experimental conditions. The degree of retention, distribution constant, as well as enthalpy and entropy values which characterize the adsorption of uranium ions on these resins were calculated. By means of Langmuir relation, taking into account the experimental data, the maximum adsorption capacity, Langmuir constant and Gibbs free energy values for the studied processes are established.
Final purification of the dissolved precipitate employed a U/TEVA·SpecTH (dipentyl pentylphosphonate) extraction chromatographic column. Studies of matrix effects upon the uptake of uranium by the DiphonixTH led to an initial dilution of the waste solution and to a reduction of ferric ion.
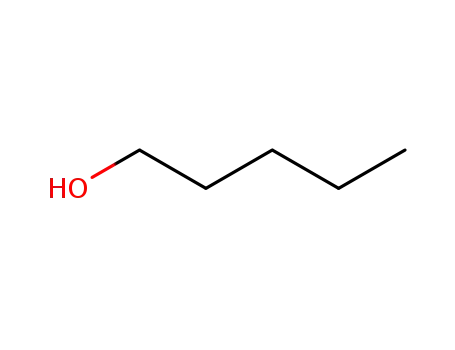
pentan-1-ol

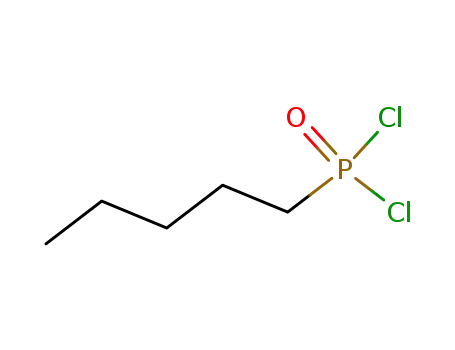
pentylphosphonic dichloride

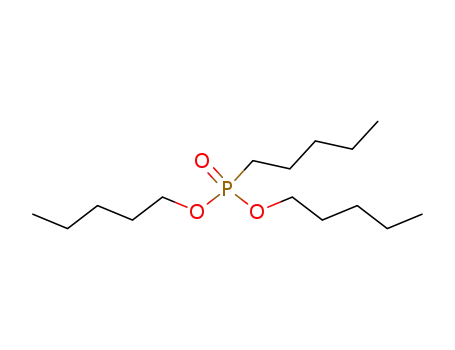
pentyl-phosphonic acid dipentyl ester
| Conditions | Yield |
|---|---|
|
|

pentan-1-ol

pentylphosphonic dichloride