
Antioxidant BHT 264
CAS:128-37-0
Purity:99%
Contact Now
We will contact you as soon as possible
Your Location:Home >Products >Pharmaceutical >14441-90-8
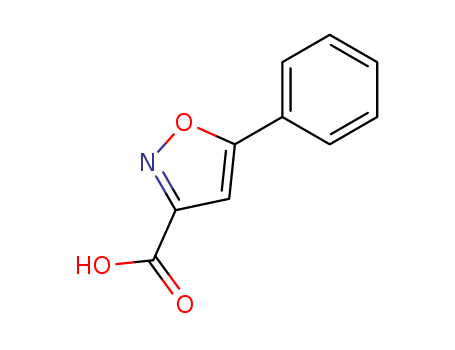

Product Details
5-Phenylisoxazole-3-carboxylic acid has been shown to inhibit the growth of tuberculosis bacteria. Some derivatives of 5-phenylisoxazole-3-carboxylic acid have been synthesized and analyzed for their ability to inhibit xanthine oxidase.
InChI:InChI=1/C10H7NO3/c12-10(13)8-6-9(14-11-8)7-4-2-1-3-5-7/h1-6H,(H,12,13)/p-1
A number of 5-phenylisoxazole-3-carboxylic acid derivatives (5a–e, 11a–e) were synthesized and analyzed for their ability to inhibit xanthine oxidase. Most of the compounds exhibited potency levels in the micromolar/submicromolar range.
New derivatives of triazole-isoxazole we...
To discover antifungal compounds with br...
The complex pathophysiology of Alzheimer...
A dehydrohalogenative approach for isoxa...
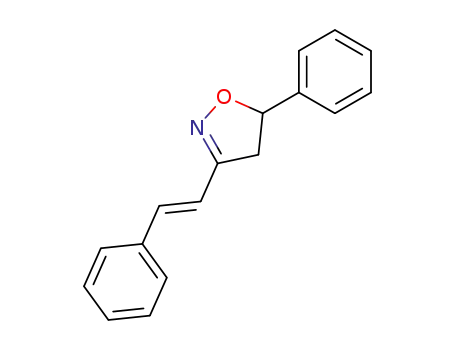
(E)-5-phenyl-3-(2-phenylethenyl)-2-isoxazoline

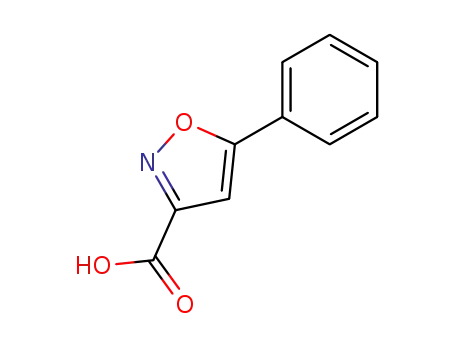
5-phenyl-3-isoxazolecarboxylic acid

benzoic acid
| Conditions | Yield |
|---|---|
|
(E)-5-phenyl-3-(2-phenylethenyl)-2-isoxazoline; With tert.-butylhydroperoxide; iron(III) chloride hexahydrate; In water; for 1h; Sealed tube;
With water; sodium hydroxide; at 80 ℃; for 10h; Sealed tube;
|
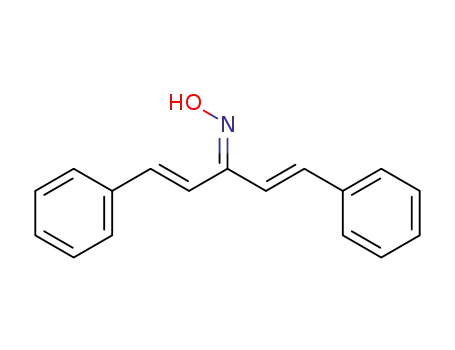
(1E,4E)-1,5-diphenylpenta-1,4-dien-3-one oxime


5-phenyl-3-isoxazolecarboxylic acid


benzoic acid
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 3 steps
1.1: copper diacetate; sodium sulfate; pyridine / 1,2-dichloro-ethane / 12 h / 25 °C
2.1: iron(III) chloride hexahydrate; 1,10-Phenanthroline / tetrahydrofuran / 80 °C / Inert atmosphere; Sealed tube
3.1: tert.-butylhydroperoxide; iron(III) chloride hexahydrate / water / 1 h / Sealed tube
3.2: 10 h / 80 °C / Sealed tube
With pyridine; tert.-butylhydroperoxide; 1,10-Phenanthroline; iron(III) chloride hexahydrate; copper diacetate; sodium sulfate; In tetrahydrofuran; water; 1,2-dichloro-ethane;
|
|
|
Multi-step reaction with 3 steps
1.1: copper diacetate; sodium sulfate; pyridine / 1,2-dichloro-ethane / 12 h / 25 °C
2.1: iron(III) chloride hexahydrate; 1,10-Phenanthroline / tetrahydrofuran / 80 °C / Inert atmosphere; Sealed tube
3.1: tert.-butylhydroperoxide; iron(III) chloride hexahydrate / water / 1 h / Sealed tube
3.2: 10 h / 80 °C / Sealed tube
With pyridine; tert.-butylhydroperoxide; 1,10-Phenanthroline; iron(III) chloride hexahydrate; copper diacetate; sodium sulfate; In tetrahydrofuran; water; 1,2-dichloro-ethane;
|
|
|
Multi-step reaction with 3 steps
1.1: copper diacetate; sodium sulfate; pyridine / 1,2-dichloro-ethane / 12 h / 25 °C
2.1: iron(III) chloride hexahydrate; 1,10-Phenanthroline / tetrahydrofuran / 80 °C / Inert atmosphere; Sealed tube
3.1: tert.-butylhydroperoxide; iron(III) chloride hexahydrate / water / 1 h / Sealed tube
3.2: 10 h / 80 °C / Sealed tube
With pyridine; tert.-butylhydroperoxide; 1,10-Phenanthroline; iron(III) chloride hexahydrate; copper diacetate; sodium sulfate; In tetrahydrofuran; water; 1,2-dichloro-ethane;
|
|
|
Multi-step reaction with 3 steps
1.1: copper diacetate; sodium sulfate; pyridine / 1,2-dichloro-ethane / 12 h / 25 °C
2.1: iron(III) chloride hexahydrate; 1,10-Phenanthroline / acetonitrile / 80 °C / Inert atmosphere; Sealed tube
3.1: tert.-butylhydroperoxide; iron(III) chloride hexahydrate / water / 1 h / Sealed tube
3.2: 10 h / 80 °C / Sealed tube
With pyridine; tert.-butylhydroperoxide; 1,10-Phenanthroline; iron(III) chloride hexahydrate; copper diacetate; sodium sulfate; In water; 1,2-dichloro-ethane; acetonitrile;
|
|
|
Multi-step reaction with 3 steps
1.1: copper diacetate; sodium sulfate; pyridine / 1,2-dichloro-ethane / 12 h / 25 °C
2.1: iron(III) chloride hexahydrate; 1,10-Phenanthroline / tetrahydrofuran / 80 °C
3.1: tert.-butylhydroperoxide; iron(III) chloride hexahydrate / water / 1 h / Sealed tube
3.2: 10 h / 80 °C / Sealed tube
With pyridine; tert.-butylhydroperoxide; 1,10-Phenanthroline; iron(III) chloride hexahydrate; copper diacetate; sodium sulfate; In tetrahydrofuran; water; 1,2-dichloro-ethane;
|
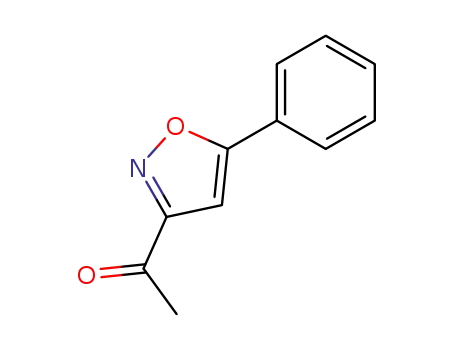
3-acetyl-5-phenyl-isoxazole
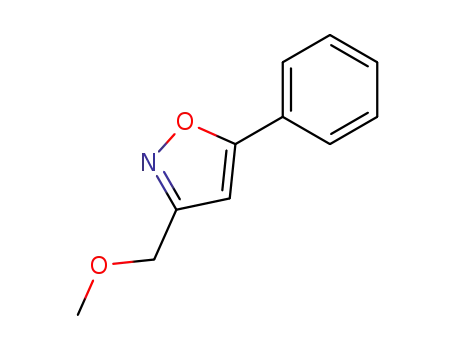
3-(methoxymethyl)-5-phenylisoxazole
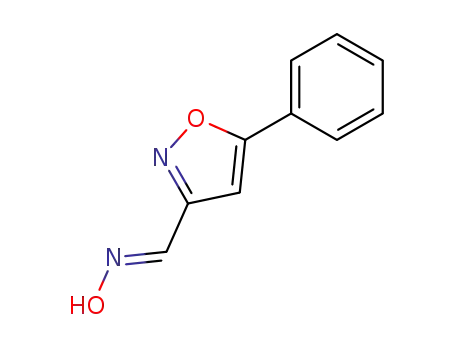
5-phenyl-isoxazole-3-carbaldehyde oxime
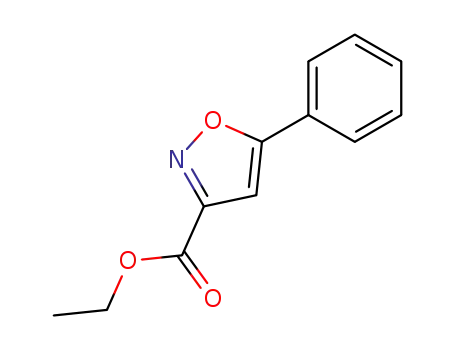
ethyl 5-phenylisoxazole-3-carboxylate
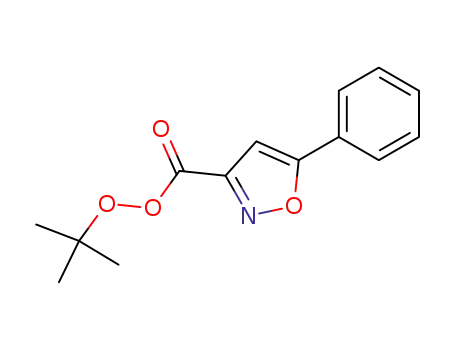
5-phenyl-isoxazole-3-carboperoxoic acid tert-butyl ester
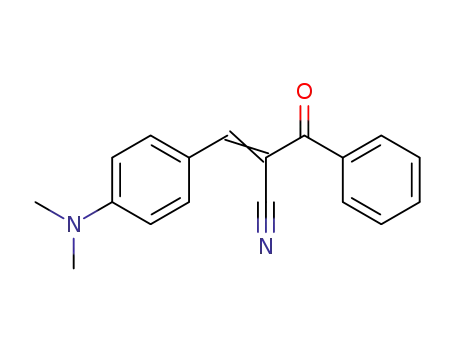
2-benzoyl-3-(4-(dimethylamino)phenyl)acrylonitrile
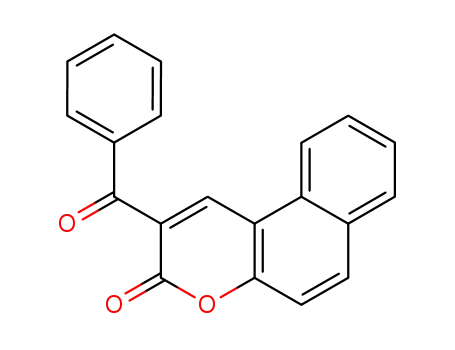
2-benzoyl-3H-benzo[f]chromen-3-one
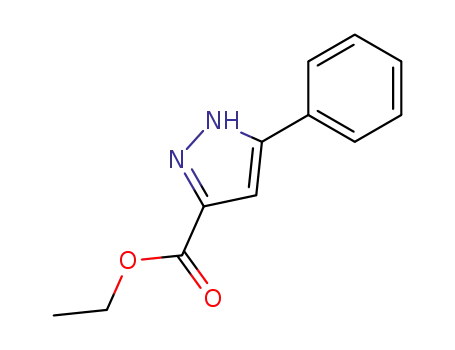
ethyl 5-phenylpyrazole-3-carboxylate