
Antioxidant BHT 264
CAS:128-37-0
Purity:99%
Contact Now
We will contact you as soon as possible
Your Location:Home >Products >Analytical Chemistry >1445-75-6
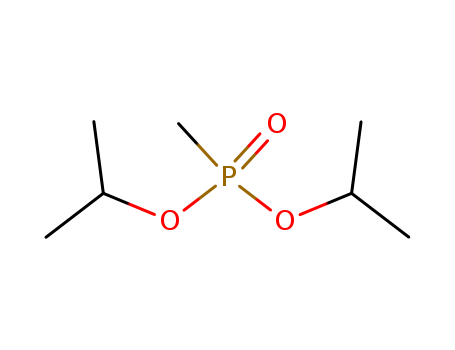

Product Details
|
Uses |
Diisopropyl methylphosphonate is used as a precursor to prepare beta-keto phosphonates. It acts as an intermediate for Horner-Wadsworth-Emmons olefination of carbonyl compounds. |
|
Definition |
ChEBI: An organic phosphonate that is the diisopropyl ester of methylphosphonic acid. |
InChI:InChI=1/C7H17O3P/c1-5(2)7(6(3)4)11(8,9)10/h5-7H,1-4H3,(H2,8,9,10)/p-2
The average solution aggregation state o...
The first solvent- and catalyst-free pro...
A liquid-liquid phase-transfer-catalyzed...
Described herein is a flexible modular a...
It is well-known that the P-acids includ...
Phosphonate conjugates, preferably, bisp...
Targeting indoleamine 2,3-dioxygenase 1 ...
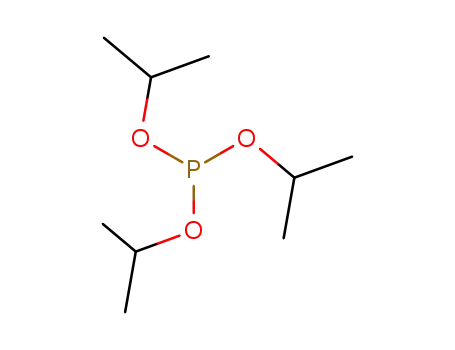
triisopropyl phosphite

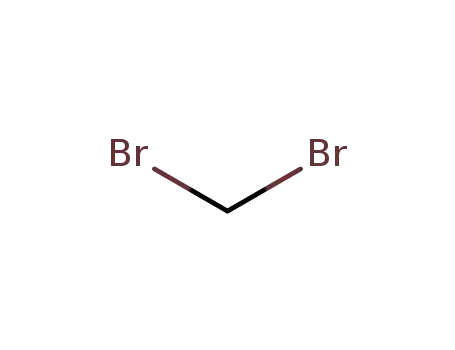
1,1-dibromomethane

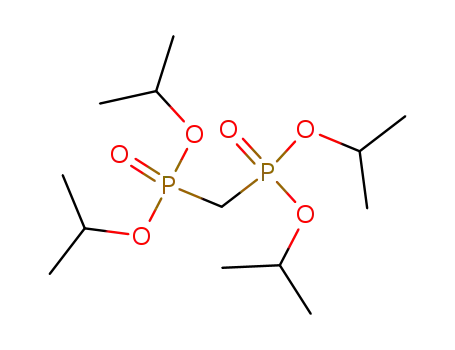
Tetraisopropyl methylenediphosphonate

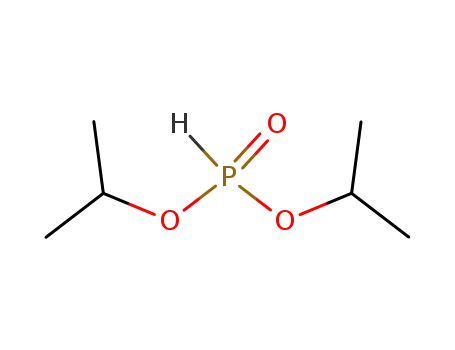
diisopropyl hydrogenphosphonate

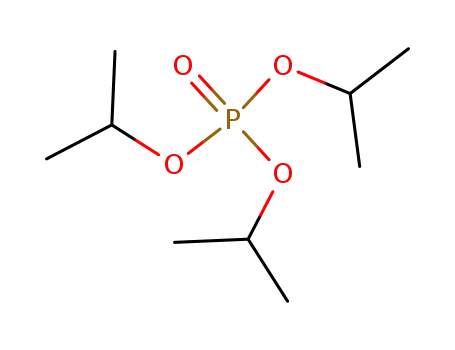
triisopropyl phosphate

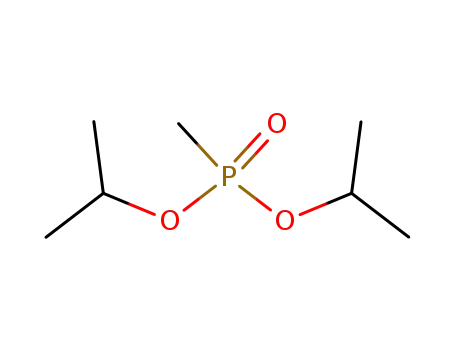
diisopropyl methanephosphonate

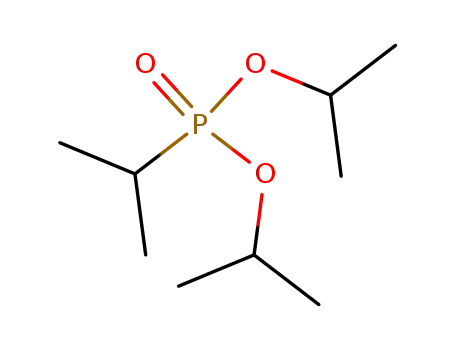
diisopropyl isopropylphosphonate

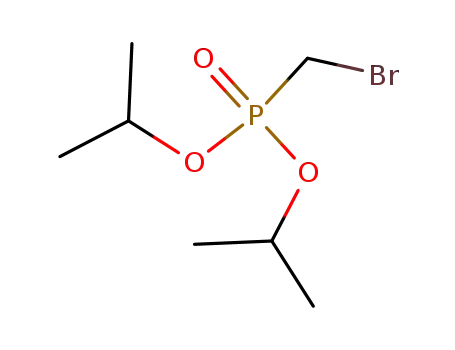
diisopropyl (bromomethyl)phosphonate
| Conditions | Yield |
|---|---|
|
In
ethylbenzene; xylene;
at 125 ℃;
for 24h;
under 760.051 - 2052.96 Torr;
Product distribution / selectivity;
|
50% |

triisopropyl phosphite

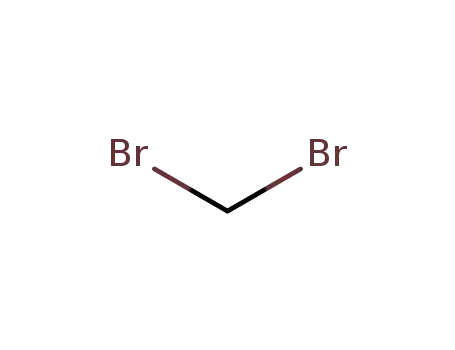
1,1-dibromomethane


Tetraisopropyl methylenediphosphonate


triisopropyl phosphate


diisopropyl methanephosphonate


diisopropyl isopropylphosphonate

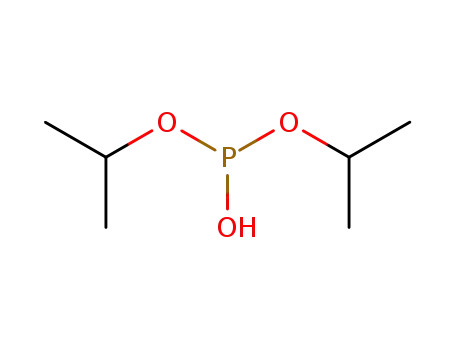
diisopropyl phosphite


diisopropyl (bromomethyl)phosphonate
| Conditions | Yield |
|---|---|
|
In
toluene;
at 120 ℃;
for 24h;
under 760.051 - 2259.82 Torr;
|
50% |
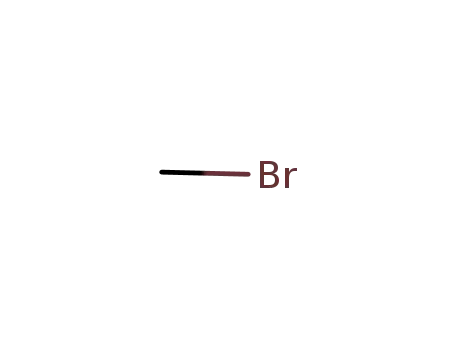
methyl bromide

triisopropyl phosphite
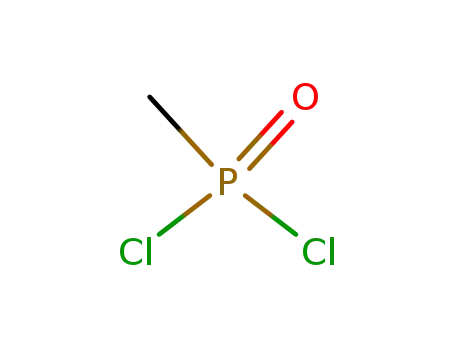
methylphosphonic acid dichloroanhydride
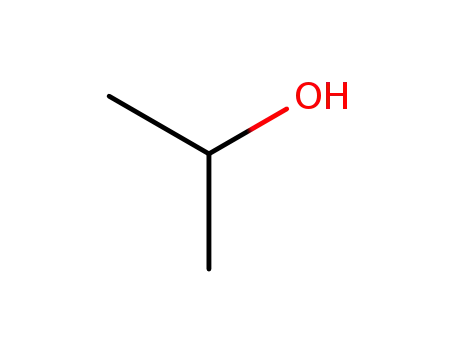
isopropyl alcohol
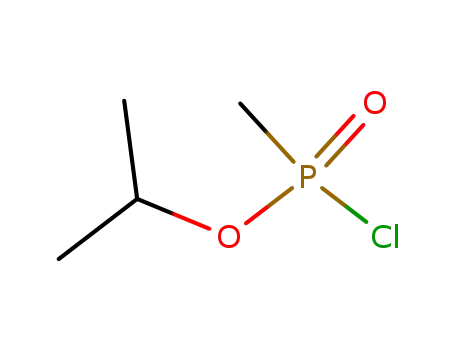
methylphosphonic chloride isopropyl ester

methylphosphonic acid dichloroanhydride
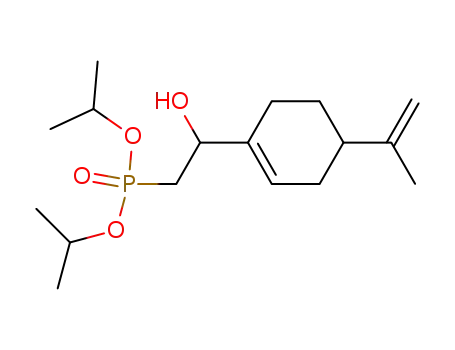
[2-Hydroxy-2-(4-isopropenyl-cyclohex-1-enyl)-ethyl]-phosphonic acid diisopropyl ester
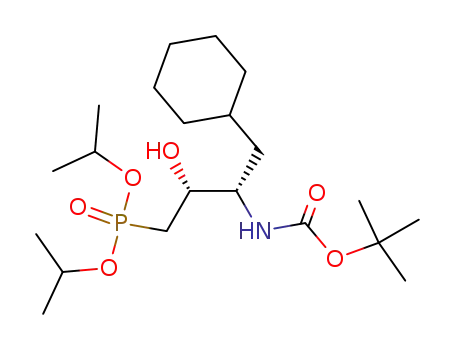
(2R,3S)-diisopropyl <3-(t-Boc-amino)-4-cyclohexyl-2-hydroxybutyl>phosphonate