
Antioxidant BHT 264
CAS:128-37-0
Purity:99%
Contact Now
We will contact you as soon as possible
Your Location:Home >Products >Chemical Reagents >78-32-0
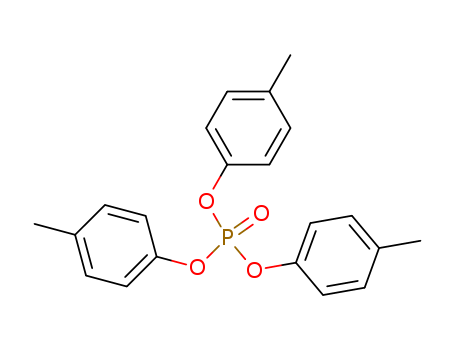

Product Details
|
Chemical Properties |
WHITE TO LIGHT BROWN POWDER |
|
General Description |
Crystalline solid. |
|
Air & Water Reactions |
Insoluble in water. |
|
Reactivity Profile |
Organophosphates, such as TRI-P-TOLYL PHOSPHATE, are susceptible to formation of highly toxic and flammable phosphine gas in the presence of strong reducing agents such as hydrides. Partial oxidation by oxidizing agents may result in the release of toxic phosphorus oxides. |
|
Health Hazard |
SYMPTOMS: Nausea, vomiting, abdominal pain, diarrhea. After 3-28 days incubation period: non-tender enlargement of parotid gland; coldness and sweating of hands and legs; muscular pain and cramping of hands and legs; parethesias of extremities; muscular tremor; symmetrical polyneuritis. |
|
Fire Hazard |
Flash point data for TRI-P-TOLYL PHOSPHATE are not available, however, TRI-P-TOLYL PHOSPHATE is probably combustible. |
InChI:InChI=1/C21H21O4P/c1-16-4-10-19(11-5-16)23-26(22,24-20-12-6-17(2)7-13-20)25-21-14-8-18(3)9-15-21/h4-15H,1-3H3
Industrially important triaryl phosphite...
We describe the synthesis of an ionic-li...
The amination of triaryl phosphates was ...
The reaction of hydroxide ion with di-4-...
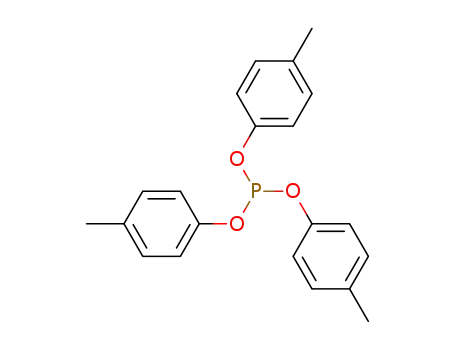
tri-p-tolylphosphite

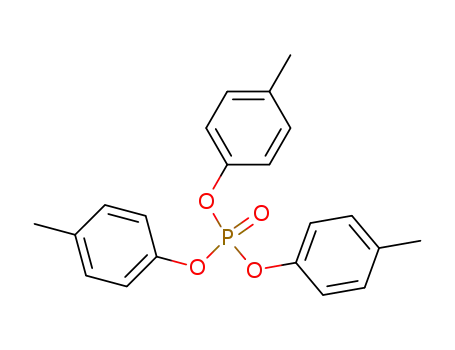
tri-p-cresyl phosphate
| Conditions | Yield |
|---|---|
|
With
1-methyl-3-(4-((2,4,6-triisopropylphenyl)tellanyl)benzyl)-1H-imidazol-3-ium hexafluorophosphate; Rose Bengal lactone;
at 15 ℃;
for 2.5h;
Irradiation;
Ionic liquid;
|
95% |
|
With
dihydrogen peroxide;
In
dimethyl sulfoxide; toluene;
at 20 ℃;
for 0.5h;
Schlenk technique;
|
92% |
|
With
1,1-diphenylethyl hydroperoxide;
In
benzene;
at 20 ℃;
Rate constant;
|
|
|
Multi-step reaction with 2 steps
1: absolute diethyl ether; chlorine
2: water
With
diethyl ether; water; chlorine;
|
|
|
With
bis(2,4,6-triisopropylphenyl) telluride; oxygen; rose bengal;
In
acetonitrile;
at 40 - 50 ℃;
for 8h;
Irradiation;
|
100 %Spectr. |
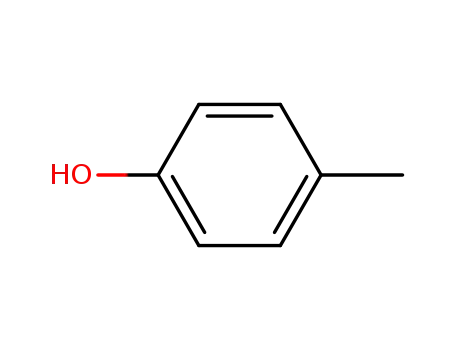
p-cresol


tri-p-cresyl phosphate
| Conditions | Yield |
|---|---|
|
With
sodium hydroxide; trichlorophosphate;
for 2.5h;
Microwave irradiation;
|
88% |
|
With
trichlorophosphate;
|
|
|
With
phosphorus pentachloride;
Behandlung des Reaktionsprodukts mit Kali;
|
|
|
With
N,N,N,N,N,N-hexamethylphosphoric triamide;
at 228 - 231 ℃;
|
|
|
With
sodium hydroxide; trichlorophosphate;
Aliquat 336;
Yield given. Multistep reaction;
1.) H2O, 2.) CHCl3, 40 min, room temp.;
|
|
|
With
sodium hydroxide; dibenzo-18-crown-6; trichlorophosphate;
N-benzyl-N,N,N-triethylammonium chloride;
Yield given. Multistep reaction;
1.) H2O, CHCl3, 2.) H2O, CHCl3, 20 min;
|
|
|
Multi-step reaction with 2 steps
1: PCl5 / Erhitzen auf 140grad
2: water
With
phosphorus pentachloride; water;
|
|
|
With
triethylamine; trichlorophosphate;
In
toluene;
at 0 - 20 ℃;
Inert atmosphere;
|
|
|
With
magnesium; trichlorophosphate;
at 80 - 130 ℃;
|
|
|
Multi-step reaction with 2 steps
1: phosphorus; potassium phosphate; diphenyl diselenide / toluene; dimethyl sulfoxide / 4 h / 60 °C / Schlenk technique; Inert atmosphere
2: dihydrogen peroxide / toluene; dimethyl sulfoxide / 0.5 h / 20 °C / Schlenk technique
With
potassium phosphate; diphenyl diselenide; phosphorus; dihydrogen peroxide;
In
dimethyl sulfoxide; toluene;
|

p-cresol
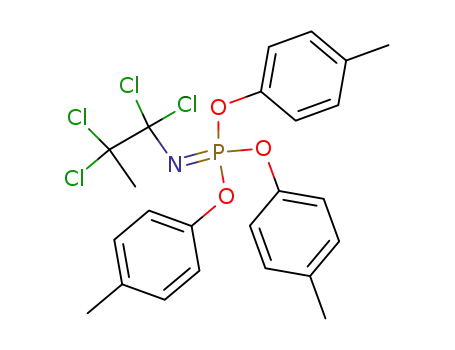
1,1,2,2-Tetrachloro-propyl-phosphorimidic acid tri-p-tolyl ester
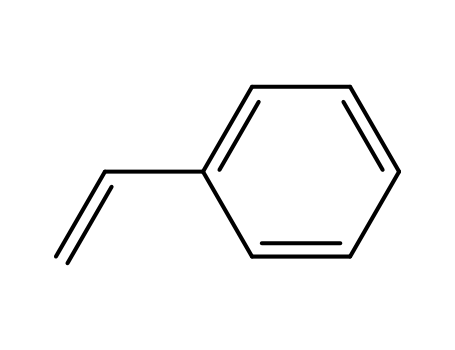
styrene
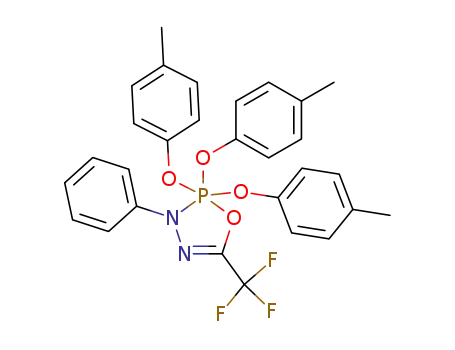
3-phenyl-5-trifluoromethyl-2,2,2-tris(4-methylphenoxy)-2,3-dihydro-1,3,4,2-oxadiazaphosphole

para-methylbenzonitrile

p-cresol
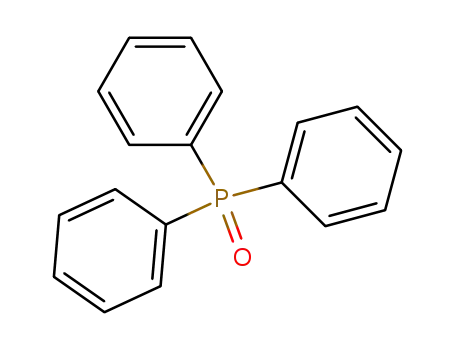
Triphenylphosphine oxide
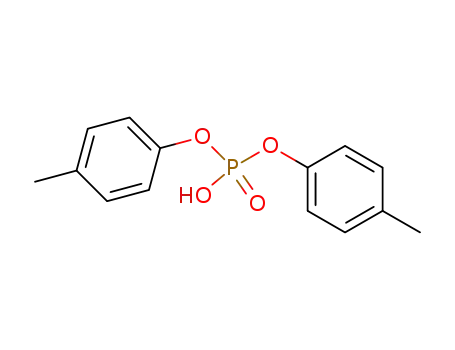
bis-(4-methylphenyl)phosphoric acid