
Antioxidant BHT 264
CAS:128-37-0
Purity:99%
Contact Now
We will contact you as soon as possible
Your Location:Home >Products >Organic Chemistry >126-71-6
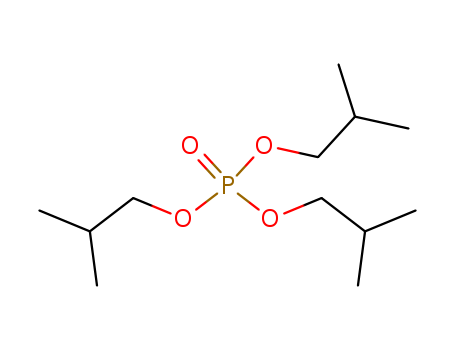

Product Details
|
Description |
Advances in technological development over the last couple of centuries have led to the use of synthetic carbon-based polymers for everyday household and office items, where once wood or metal were desired. The high fuel values for some of these materials could pose danger where risk of combustion is high; therefore, flame retardants have been introduced into and coating for electronic devices. These substances have a broad application field and good fire safety performance. |
|
Uses |
Phosphate esters are used as flame retardants, plasticizers, hydraulic fluids, solvents, extraction agents, antifoam agents, Partition behavior in water, sediment, and soil Phosphate ester flame retardants enter the environment from industrial sources and disposal of consumer products containing flame retardants. These anthropogenic compounds have been detected in water, soil, and air owing to widespread use following their fast emergence and popularization during 1970s. Occurrence of these phosphate ester flame retardants is widespread in surface water and groundwater because of the leaching of PVC plastics and polyurethane foams, effluent from industrial sources, and spills of hydraulic fluids. This primary contaminated water is then transported to a secondary source, such as drinking water. Hydrolysis, although slow because of poor solubility and pH dependence, is the most important abiotic elimination process. In soil and sediment, phosphate ester flame retardants are persistent because they have the tendency to adsorb strongly. Volatilization and biodegradation are potential elimination processes for phosphate esters adsorbed to soil. Environmental persistency (degradation/speciation) These retardants can change chemical composition in the environment. Generally, most phosphate esters are poorly soluble in water and adsorb strongly to soils. These compounds are considered emerging pollutants because of their prevalence and persistence in the environment. Particulate-phase phosphate esters are subject to wet and dry deposition, whereas semi-volatile phosphate esters have the potential to hydrolyze to diesters, monoesters, and phosphoric acid. There is no information available that suggests that selected phosphate ester flame retardants undergo transformation or degradation in the atmosphere. Long-range transport This is highly dependent on the specific compound. Bioaccumulation and biomagnification Phosphate esters are subject to biodegradation in aquatic and terrestrial environments. |
|
Flammability and Explosibility |
Notclassified |
|
Environmental Fate |
Routes and pathways relevant physicochemical properties (e.g., solubility, Pow, Henry constant.)consumer and industrial items and play an important role in safeguarding life and property. A large and diverse group of anthropogenic compounds constitute flame retardants, which are added to combustible materials to render them more resistant to ignition. They are designed to minimize the risk of a fire in the event of contact with a small heat source such as a cigarette. A wide range of different flame retardants is produced, because many materials and products that are to be rendered fire safe are very different in nature and composition. Therefore, having variety in flame retardant products is necessary so as to retain key material functionality. For example, plastics have a wide range of mechanical and chemical properties and differ in combustion behavior. These materials in particular are the main focus of phosphate ester flame retardants.Phosphate esters are derivatives of tri protic acid, phosphoric acid, with a general formula of RxH3°xPO4. Flame retardants are composed of a group of chemicals with similar properties but slightly different structures. They are typically liquids and some are solids at room temperature. Some examples of the phosphate ester flame retardants include: tris(2-chloroethyl)phosphate (TCEP), tributyl phosphate (TnBP), tris(2-butoxyethyl) phosphate (TBEP), tris(1,3-dichloro-2-propyl) phosphate (TDCP), triphenyl phosphate (TPP), tris(2-chloro-isopropyl) phosphate (TCPP), and triisobutyl phosphate (TiBP). These compounds are trisubstituted and categorized as alkyl (TnBP, TiBP), alkyl ether (TBEP), chloroalkyl (TCEP, TCPP, TDCP), and aryl (TPP) phosphate esters. |
|
Purification Methods |
Purify it by repeated crystallisation, from hexane, of its addition compound with uranyl nitrate. (see tributyl phosphate.) [Siddall J Am Chem Soc 81 4176 1959; see Cherbuliez in Organo Phosphorus Compounds (Kosolapoff & Maier eds) Wiley Vol 6 pp 211-577 1973.] |
InChI:InChI=1/C12H27O4P/c1-10(2)7-14-17(13,15-8-11(3)4)16-9-12(5)6/h10-12H,7-9H2,1-6H3
This paper presents measurements on the ...
The "third phase" formation phenomenon i...
Due to obtain trialkylphosphate is sugge...
The novel eco-efficient methods to trans...
Oxidative alkoxylation of Zn3P2 with the...
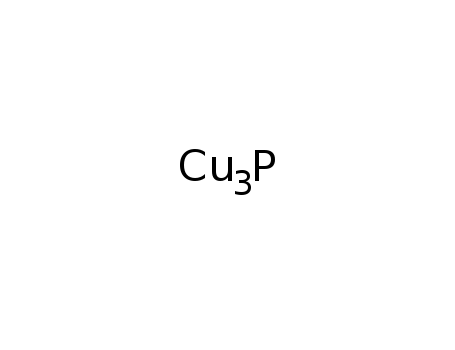
copper phosphide

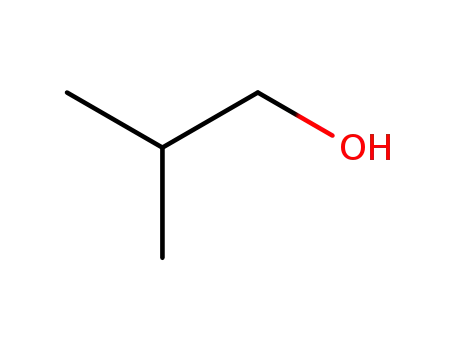
2-methyl-propan-1-ol

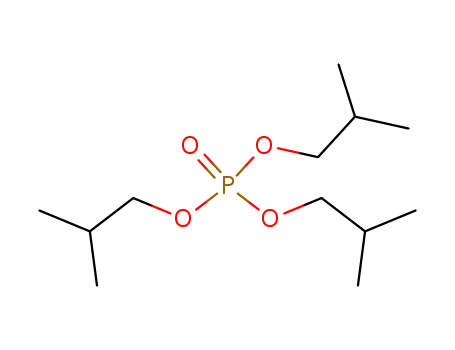
triisobutyl phosphate

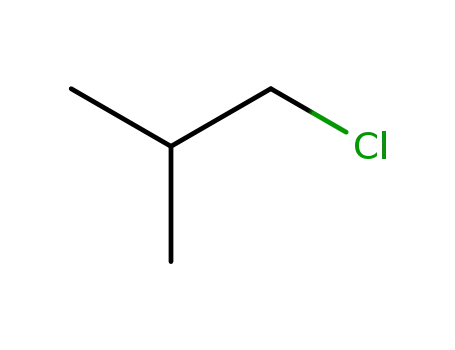
isobutyryl chloride

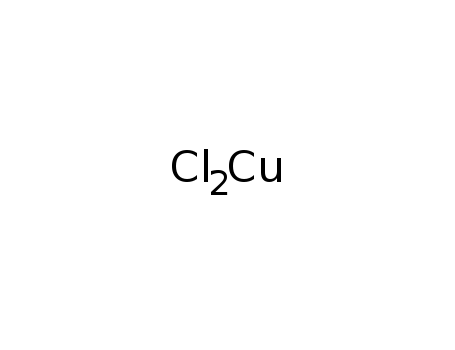
copper dichloride
| Conditions | Yield |
|---|---|
|
With
O2; CuCl2;
In
neat (no solvent);
byproducts: H2O, HCl; the alcohol was loaded together with CuCl2 into the reactor and purged with an Ar-O2 mixture; then Cu3P was added and the mixt. reacted at 293 K, 323 K or 343 K;;
Kinetics;
|
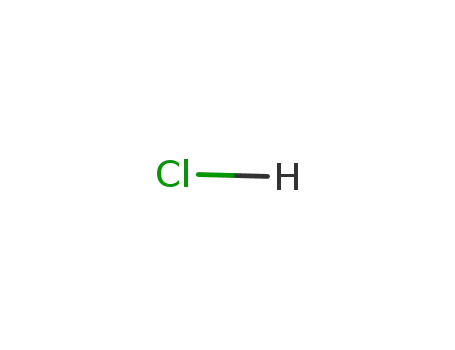
hydrogenchloride


zinc(II) phosphide


2-methyl-propan-1-ol

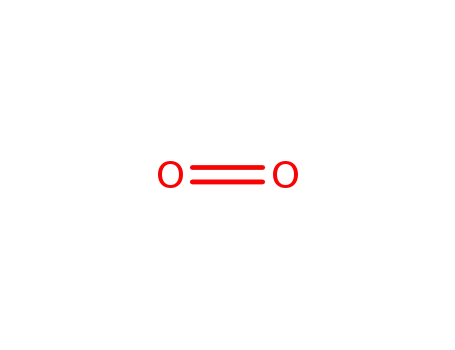
oxygen

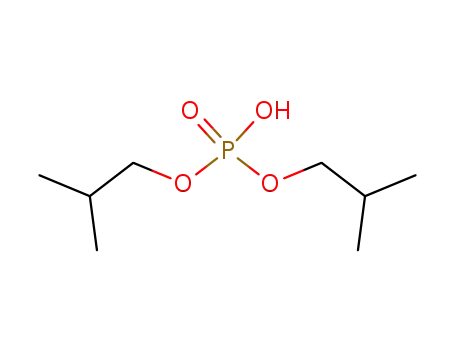
diisobutyl phosphoric acid


triisobutyl phosphate


isobutyryl chloride

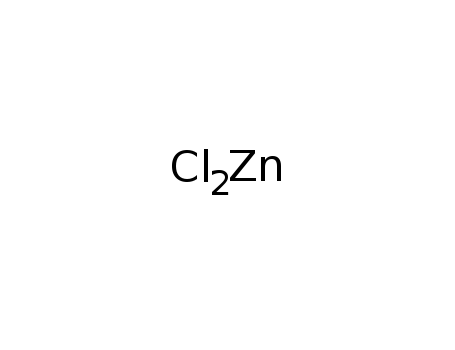
zinc(II) chloride
| Conditions | Yield |
|---|---|
|
copper dichloride;
In
further solvent(s);
Kinetics;
|

2-methyl-propan-1-ol
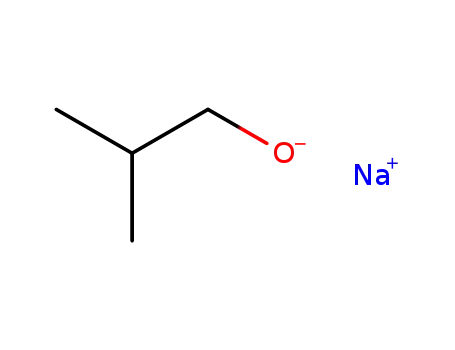
sodium isobutoxide
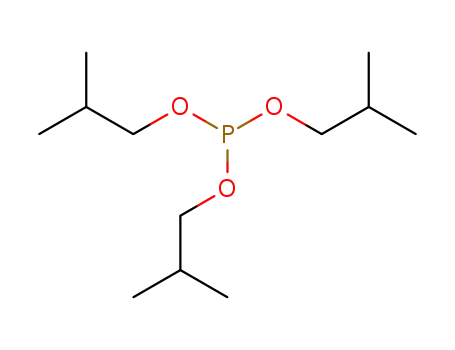
triisobutyl phosphite
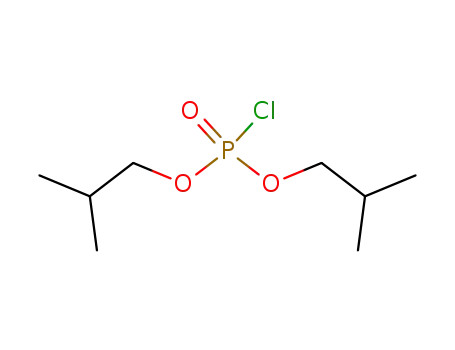
phosphorochloridic acid diisobutyl ester
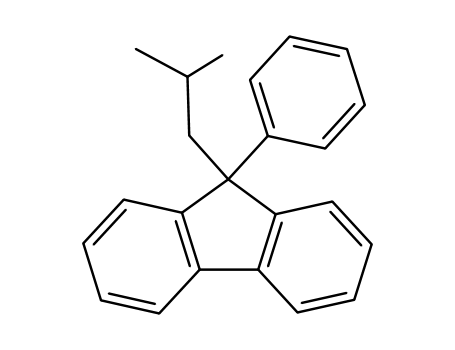
9-Isobutyl-9-phenyl-fluoren
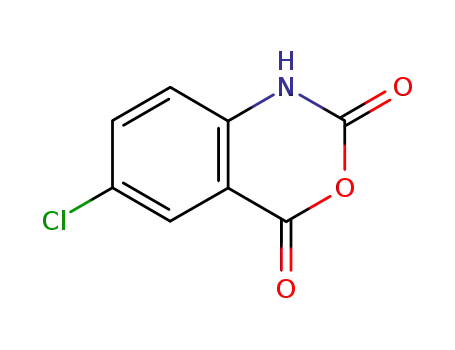
5-Chloroisatoic anhydride
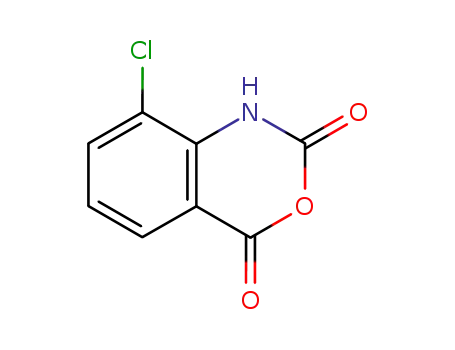
8-chloro-2H-benzo[d][1,3]oxazine-2,4(1H)-dione
B-tris(di-i-butoxy phosphoryl) borazine