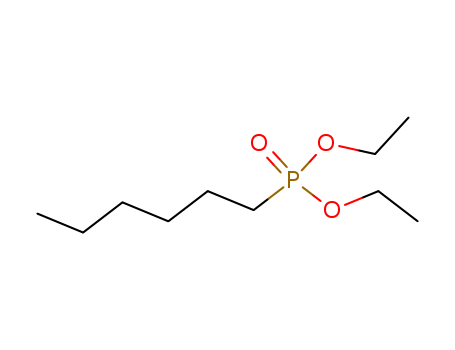
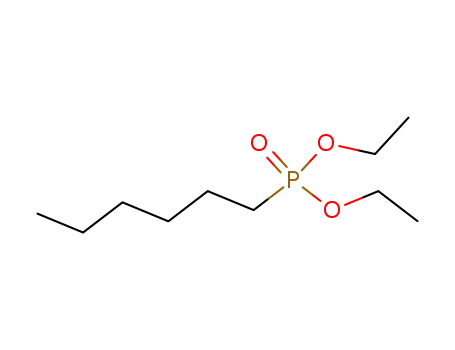
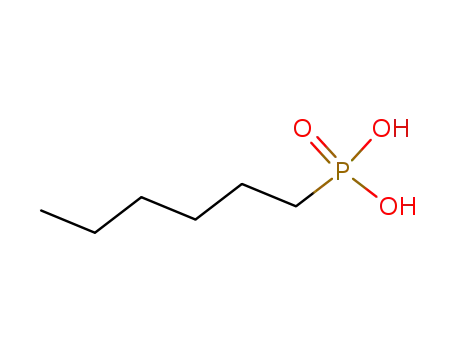
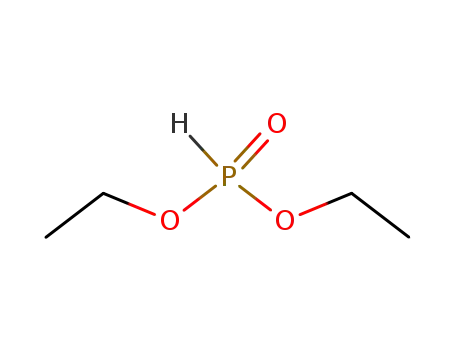
Buy High Grade hexylphosphonic acid diethyl ester, Offer 16165-66-5 with Best Price
- Molecular Formula:C10H23 O3 P
- Molecular Weight:222.265
- Vapor Pressure:0.00569mmHg at 25°C
- Refractive Index:1.4820 (estimate)
- Boiling Point:282.6°Cat760mmHg
- Flash Point:138.6°C
- PSA:45.34000
- Density:0.964g/cm3
- LogP:3.83280
hexylphosphonic acid diethyl ester(Cas 16165-66-5) Usage
|
Uses
|
Diethyl Hexylphosphonate is an intermediate in the synthesis of Hexylphosphonic Dichloride (H295465). Hexylphosphonic Dichloride acts as a reagent for the preparation of enantiomeric phosphonates which are inhibitors of cholesterol esterase.
|
InChI:InChI=1/C10H23O3P/c1-4-7-8-9-10-14(11,12-5-2)13-6-3/h4-10H2,1-3H3
16165-66-5 Relevant articles
Phosphonate inhibitors of antigen 85C, a crucial enzyme involved in the biosynthesis of the Mycobacterium tuberculosis cell wall
Gobec, Stanislav,Plantan, Ivan,Mravljak, Janez,Wilson, Rosalind A.,Besra, Gurdyal S.,Kikelj, Danijel
, p. 3559 - 3562 (2004)
The first phosphonate inhibitors of anti...
Synthesis and polymerization kinetics of acrylamide phosphonic acids and esters as new dentine adhesives
Besse,Le Pluart,Cook,Pham,Madec
, p. 149 - 157 (2013)
In restorative dentistry, acrylamide mon...
Conjugate Addition of Alkyl- and Vinyl-copper Complexes to α,β-Unsaturated Phosphonic Esters. Stereospecific Syntheses of γ,δ-Unsaturated Phosphonates
Nicotra, Francesco,Panza, Luigi,Russo, Giovanni
, p. 5 - 6 (1984)
Alkylcopper complexes add to diethyl eth...
Rotational Motions in n-Hexane Phosphonic Acid Diethyl Ester Studied Combining 2H, 13C, and 31P NMR. Analysis of the Phosphorus-31 Spin-Lattice Relaxation
Petr, A.,Grossmann, G.,Klose, G.,Ahlnaes, T.,Goetze, T.
, p. 231 - 242 (1986)
Spin-lattice relaxation of 13C and 31P i...
Direct conversion of phosphonates to phosphine oxides: An improved synthetic route to phosphines including the first synthesis of methyl JohnPhos
Kendall, Alexander J.,Salazar, Chase A.,Martino, Patrick F.,Tyler, David R.
supporting information, p. 6171 - 6178 (2015/02/19)
The synthesis of tertiary phosphine oxid...
16165-66-5 Process route
-
- 122-52-1
triethyl phosphite
-
- 16165-66-5
diethyl hexylphosphonate
Conditions
| Conditions |
Yield |
|
at 150 ℃; for 8h;
|
85% |
|
at 150 ℃; for 8h;
|
85% |
|
|
|
-
- 4721-24-8
n-hexylphosphonic acid
-
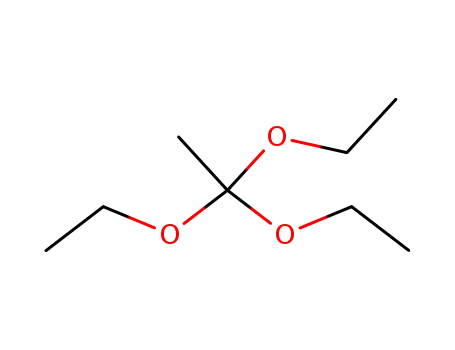
- 78-39-7
Triethyl orthoacetate
-
- 16165-66-5
diethyl hexylphosphonate
Conditions
| Conditions |
Yield |
|
at 90 ℃; for 24h; Green chemistry;
|
91% |
16165-66-5 Upstream products
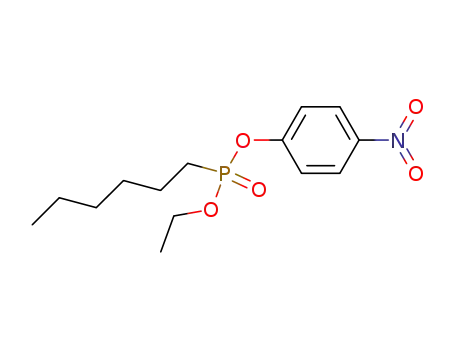
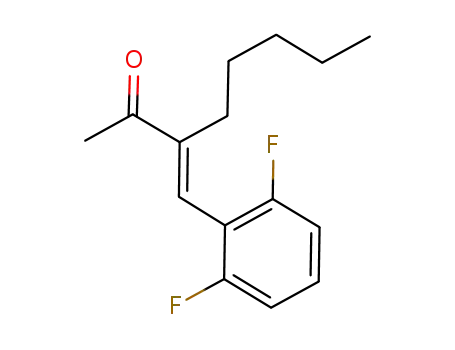
16165-66-5 Downstream products
-
3015-76-7
hexyl-phosphonic acid ethyl ester-(4-nitro-phenyl ester)
-
4721-24-8
n-hexylphosphonic acid
-
1065850-15-8
(E)-3-(2,6-difluorobenzylidene)octan-2-one
-
1065850-09-0
(E)-3-(cyclohex-3-enylmethylene)octan-2-one