
Antioxidant BHT 264
CAS:128-37-0
Purity:99%
Contact Now
We will contact you as soon as possible
Your Location:Home >Products >Inorganic chemistry >2404-58-2
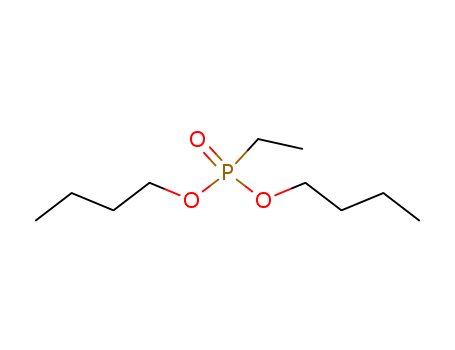

Product Details
|
Description |
Phosphonic Acid (Ethyl): The core structure consists of a phosphonic acid group with an ethyl substituent. Dibutyl Ester: The phosphonic acid is esterified with two butyl (C4H9) groups. |
It is well-known that the P-acids includ...
New P-alkyl 2,3-oxaphosphabicyclo-[2.2.2...
The invention relates to a method for pr...
The coupling reaction of alkylphosphonic...
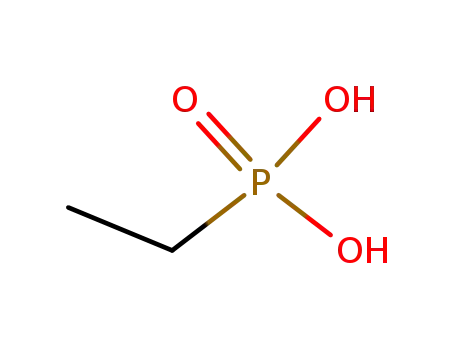
ethylphosphonic acid

butan-1-ol

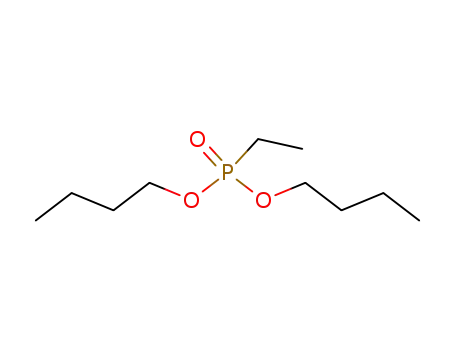
dibutyl ethylphosphonate
| Conditions | Yield |
|---|---|
|
With tetrachlorosilane; at 0 ℃;
|
91% |
|
With p-TsOH-Celite; at 20 ℃;
|
90% |
|
In toluene; at 85 - 100 ℃;
|
89% |

ethylphosphonic acid


butan-1-ol


dibutyl ethylphosphonate

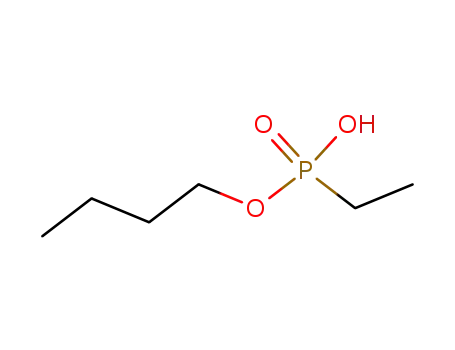
monobutyl ethylphosphonate
| Conditions | Yield |
|---|---|
|
With 1-butyl-3-methylimidazolium Tetrafluoroborate; at 180 ℃; for 4h; Temperature; Overall yield = 69 percent; Microwave irradiation; Green chemistry;
|
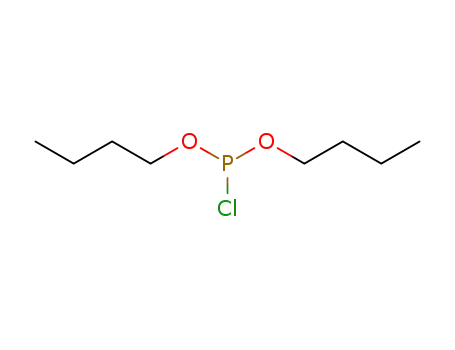
dibutyl chlorophosphite
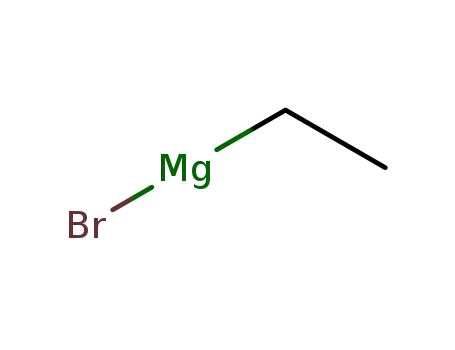
ethylmagnesium bromide
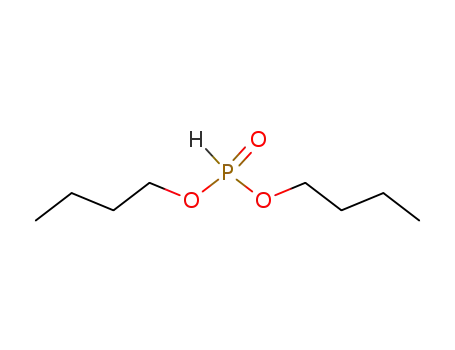
dibutyl hydrogen phosphite
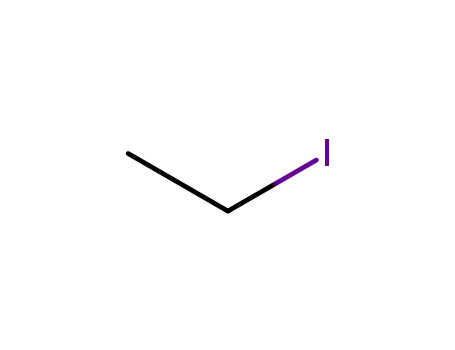
ethyl iodide
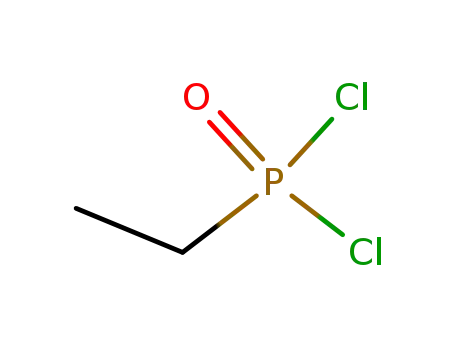
Ethylphosphonic dichloride

ethylphosphonic acid